
ASH 2025 – Regeneron’s Lynozyfic shows front-line promise
Linker-MM4 data raise more questions about sequencing of therapies for multiple myeloma.
Linker-MM4 data raise more questions about sequencing of therapies for multiple myeloma.

BCMA-targeting bispecifics could be moving even earlier in multiple myeloma, and Regeneron is joining the push, touting first-line data with Lynozyfic. Results from the phase 1/2 Linker-MM4 study are “some of the best we’ve seen with an off-the-shelf single agent in induction therapy”, said MD Anderson’s Professor Robert Orlowski, presenting at ASH on Sunday.
The field is evolving fast, though, with a combination of Johnson & Johnson’s rival product Tecvayli, plus Darzalex, apparently heading for the second line. “I think we really need more data to know which combinations are the best, and whether using it earlier or in second line is the best,” Orlowski added.
He contended that there was “room for a monotherapy”, adding that one potential benefit is less toxicity compared with a quadruplet containing Darzalex, for example. “And you’re still saving the quadruplet for relapse, if that were to become an issue.”
Still, Lynozyfic has toxicity of its own. And it could be a while before doctors will have to make such a choice: Lynozyfic’s pivotal first-line trial, Linker-MM, is only just getting under way. That study is adding the anti-BCMA T-cell engager to induction with Darzalex, Revlimid and dexamethasone, versus continued therapy with the triplet.
Linker-MM4
Linker-MM4 had a different, “window-of-opportunity” design, with patients given Lynozyfic monotherapy initially, then moved to standard of care if they didn’t show at least a partial response by cycle two, and a very good partial response by cycle four. Regeneron claimed it's the first clinical trial to evaluate a BCMA-targeting bispecific as monotherapy in newly diagnosed disease.
The study enrolled transplant-ineligible and transplant-eligible patients alike, with the latter also able to receive autologous stem cell transplant.
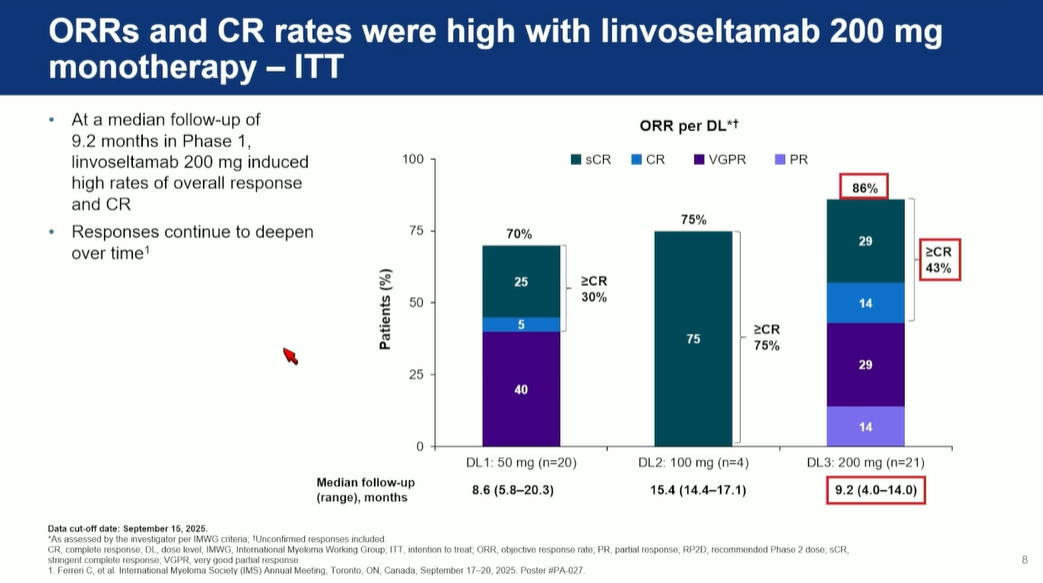
Orlowski highlighted the go-forward dose of 200mg, where there was an ORR of 86% among 21 patients in phase 1, at a cutoff date of 15 September. He noted that this was a stringent intent-to-treat analysis, which included patients who were moved off therapy, and that follow-up time was relatively short, so he postulated that responses could improve.
However, this figure encompassed investigator-assessed responses, and also included unconfirmed, as well as confirmed responses – both facts might flatter Lynozyfic.
Orlowski noted no new safety signals for Lynozyfic, which already sports a black box warning for cytokine release syndrome and neurotoxicity including ICANS in its label for fifth-line multiple myeloma.
In Linker-MM4, 91% of patients had treatment-related adverse events, 67% of these at grade 3 or 4. More reassuringly, there were no grade 5 treatment-emergent adverse events, or dose-limiting toxicities, and only one patient experienced grade 1 ICANS, with a 50mg dose.
The phase 2 portion of Linker-MM4 is now under way, testing 200mg. As well as the first-line Linker-MM6 trial, Regeneron is studying Lynozyfic as early as in the second line, in Linker-MM3. Here it could ultimately go up against Tecvayli and Darzalex, which according to an ASH late-breaking abstract reduced the risk of progression or death by 83% versus Darzalex plus dexamethasone and either Pomalyst or Velcade.
Various phase 3 studies of BCMA-targeting bispecifics are also ongoing in earlier multiple myeloma settings.
Phase 3 trials of Lynozyfic in multiple myeloma
| Trial | Setting | Regimen | Note |
|---|---|---|---|
| Linker-MM3 | r/r (1-4 prior lines) | Monotx, vs EPd | Enrolment completed Q1 2025, primary completion Apr 2033 |
| Linker-MM5 | r/r (1-3 prior lines) | +/- Kyprolis, vs SOC combos | To start Jan 2026 |
| Linker-MM6/ EMN39* | 1st-line (transplant-ineligible) | DRd induction + Lynozyfic maintenance, vs continued DRd maintenance | To start Dec 2025 |
Notes: *investigator-sponsored; D=Darzalex; d=dexamethasone; E=Empliciti; P=Pomalyst. Source: OncologyPipeline & clinicaltrials.gov.
1993













