
ASCO-GI – another surprising TIGIT success?
Skyscraper-08 yields a positive result that – with caveats – looks decent on a cross-trial basis too.
Skyscraper-08 yields a positive result that – with caveats – looks decent on a cross-trial basis too.

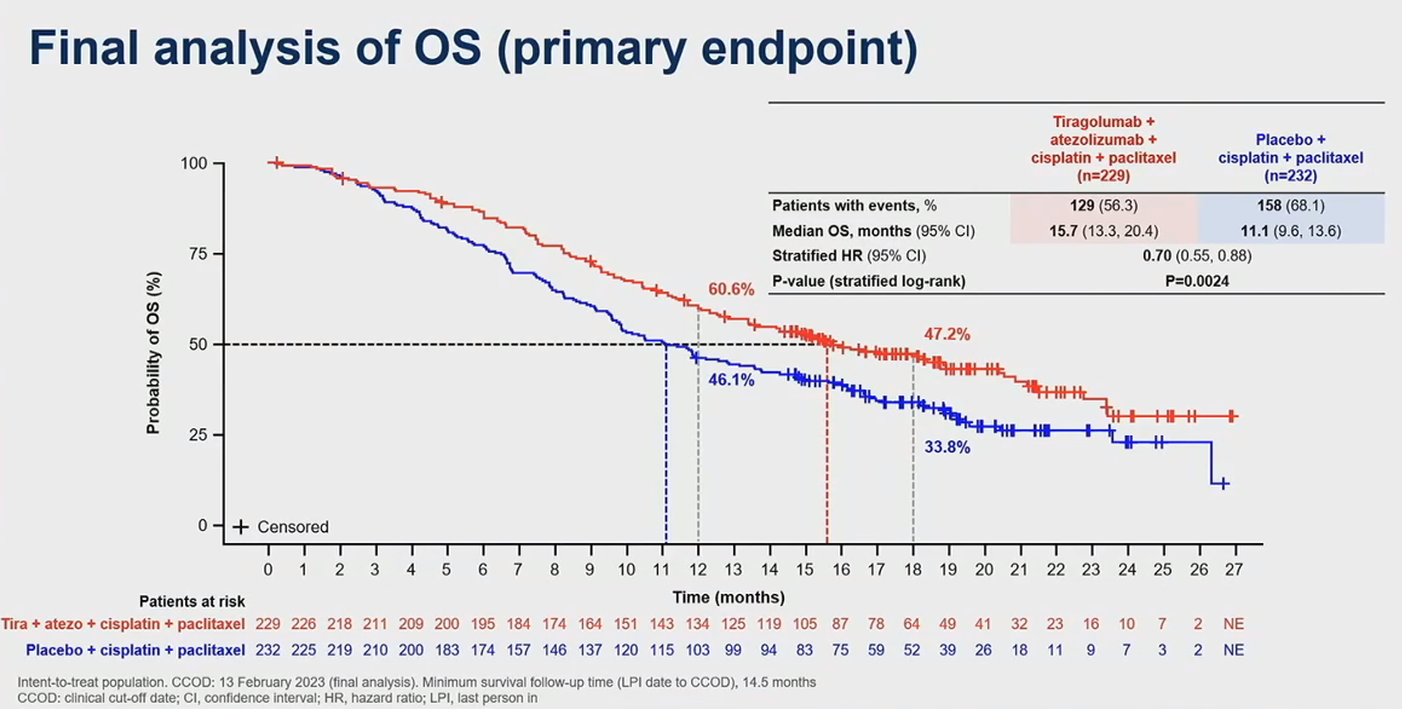
The big surprise about Skyscraper-08, the long delayed Roche PD-L1/TIGIT combo trial in front-line oesophageal squamous cell carcinoma, is that it hasn’t failed. Data unveiled at the ASCO Gastrointestinal Cancers Symposium have revealed that Tecentriq plus tiragolumab beat chemo on both co-primary endpoints of overall and progression-free survival.
However, this win comes with a couple of major caveats, most notably that Skyscraper-08 used an outdated control arm of chemotherapy alone. Still, cross-trial comparisons suggest that the topline result looks at least as good as rival studies backing chemo combos of Keytruda and Opdivo in front-line oesophageal cancer.
No doubt the result will be picked apart at length after its presentation at ASCO-GI today. Skyscraper-08 was an Asia-only trial, and the baseline differences between Asian and western gastrointestinal cancer patients make cross-trial comparisons especially tricky.
That said, the absolute median overall and progression-free survival numbers for Roche’s TIGIT combo are numerically higher than those Bristol Myers Squibb reported from Checkmate-648 backing a combination of Opdivo plus chemo in a similar first-line population of patients with squamous histology.
The numbers also look competitive against Merck’s Keynote-590 trial of Keytruda plus chemo. However, this comparison is complicated further by the fact that Keynote-590 included squamous and non-squamous histologies, as well as cancers of the gastroesophageal junction.
Cross-trial comparisons in oesophageal squamous cell carcinoma.
| Checkmate-648* | Keynote-590** | Skyscraper-08^ | ||||
|---|---|---|---|---|---|---|
| Opdivo + chemo | Chemo | Keytruda + chemo | Chemo | Tecentriq + tiragolumab | Chemo | |
| mOS (months) | 13.2 | 10.7 | 12.4 | 9.8 | 15.7 | 11.1 |
| mPFS (months) | 5.8 | 5.6 | 6.3 | 5.8 | 6.2 | 5.4 |
| ORR | 47% | 27% | 45% | 29% | 60% | 46% |
Notes: *Checkmate-648 also included an Opdivo + Yervoy cohort, which yielded less impressive numbers but which has also been US approved; **Keynote-590 included various histologies, as well as gastroesophageal junction cancer; ^two-sided alpha allocation in Skyscraper-08 was split between OS (0.04) and PFS (0.01). Source: ASCO-GI & prescribing information.
For those seeking to pick holes in Roche’s data, one obvious point is that Skyscraper-08’s chemo control arm appears to have outperformed those in Checkmate-648 and Keynote-590, on overall survival as well as on remission rates.
One interpretation of this is that the Asian patients enrolled into Skyscraper-08 had better prognosis than those in Bristol and Merck’s global trials. If that’s the case then it calls into question the value of Tecentriq plus tiragolumab’s 15.7 months of median OS, which might be expected to be somewhat lower in a poorer-prognosis population.
A separate conundrum is why it has taken so long to report data from Skyscraper-08; the study was initially expected to end in August 2024, but this was brought forward to February 2023. This implied early completion – indeed, the ASCO-GI abstract cites a February 2023 cutoff for the OS analysis – but Roche didn’t reveal anything about the results for the rest of last year, prompting at least one sellside analyst to speculate failure.
Roche itself had played down the importance of Skyscraper-08 during an investor call last year. However, the TIGIT space remains hot, and despite the ongoing delays to the reporting of final OS data from the event-driven Skyscraper-01 trial in first-line lung cancer, the markets remain upbeat about this readout’s prospects in light of the numbers revealed at first and second interim analyses.
At last year’s ASCO conference Roche’s TIGIT combo scored an unexpected success in front-line liver cancer in the Morpheus-liver trial, on the back of which the pivotal Imbrave-152/Skyscraper-14 trial has been initiated. The Morpheus-liver win came with important caveats, and Skyscraper-08 will only add to the intrigue.
This is an updated version of a story published earlier.
2978













