
CytomX's EpCAM pivot does the job
After earlier masked probody setbacks, CytomX surprises with CX-2051.
After earlier masked probody setbacks, CytomX surprises with CX-2051.

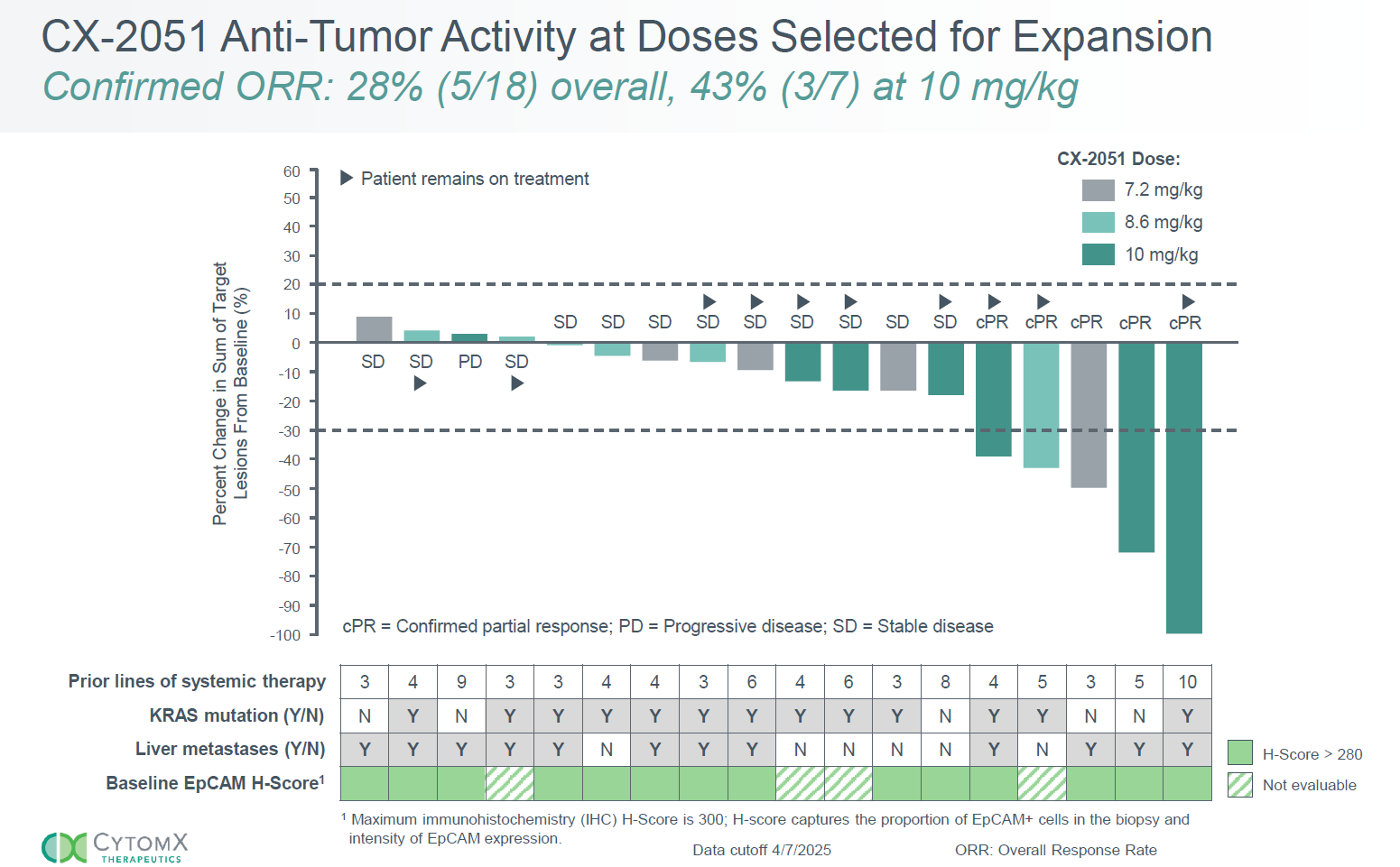
Just four months after pivoting to CX-2051, a masked antibody-drug conjugate against EpCAM, CytomX has delivered. Promising clinical data in a small number of late-line colorectal cancer patients treated with CX-2051, revealed on Monday, have enabled the company to close a $100m equity offering on the back of a share price that doubled overnight.
True, for now just 18 patients are involved, but that's probably the biggest caveat about the results, from a phase 1 study that aims to enrol 124 subjects across various tumour types. The headline number is a 28% confirmed response rate from patients given doses of 7.2mg/kg, 8.6mg/kg or 10.0mg/kg at a 7 April cutoff.
Perhaps the most promising sign is that the highest dose did best, delivering three of the five PRs. Just one of the five responders has relapsed with progressive disease, about four months after first responding. One other has come off while in response, while another subject, with stable disease, remains on trial at 9.5 months.
Single-digit norm
The colorectal cancer cohort comprises third-line or later patients, where CytomX says the likes of Fruzaqla and Stivarga deliver single-digit response rates. 64% of the patients had liver metastases, and the same proportion had KRAS-mutated disease; nearly all were microsatellite-stable, meaning that they weren't candidates for checkpoint blockade.
Importantly, they hadn't been preselected for EpCAM expression. The company says this protein is expressed at high levels in colorectal cancer, as well as in "most solid tumours", but that the problem in targeting EpCAM has stemmed from its expression also on healthy tissue. This has caused off-tumour side effects, notably pancreatitis, and made EpCAM an unpopular development target.
Against this backdrop CytomX boasted an absence of pancreatitis, grade 4 or 5 events, or indeed of any dose-limiting toxicities. Serious AEs did occur in five subjects, including grade 3 diarrhoea and acute kidney injury; prophylaxis with loperamide has been implemented to mitigate the former. There were dose delays and reductions, but CytomX said these didn't stop patients remaining on study.
The company aims to expand the three dose cohorts highlighted to 20 patients each, and says one of these is likely to comprise the recommended go-forward dose.
The trial included 2.4mg/kg and 4.8mg/kg cohorts (one patient in each), doses deemed sub-therapeutic, as well as two doses above 10.0mg/kg; while the high-dose cohorts were in March said to be enrolling, CytomX has said nothing about any efficacy or safety data they have yielded. Five 7.2-10.0mg/kg patients (one early progresser and four pre-scan) weren't efficacy evaluable.
The area of masked therapeutics, whose purpose is to deliver a probody with active domains that remain masked, to reduce activity in normal tissues, before these domains are cleaved off by proteases in the tumour microenvironment, remains contentious.
Xilio has twice flirted with insolvency before being rescued by licensing partners, while Janux is sitting on a $1.5bn valuation despite repeatedly delivering datasets that include a significant amount of curation. CytomX itself has suffered numerous setbacks, and only its repeated ability to score licensing deals has allowed it to tread water.
In March CytomX and its partner Amgen decided to discontinue the anti-EGFR T-cell engaging probody CX-904, two months after this was deprioritised and CytomX pivoted to CX-2051. More data on CX-2051 are needed to cement the victory, but for now that looks to have been a smart move.
3659













