DLL3 goes trispecific
Suzhou Zelgen’s DLL3 x DLL3 project looks better than regular T-cell engagers, with caveats.
Suzhou Zelgen’s DLL3 x DLL3 project looks better than regular T-cell engagers, with caveats.

DLL3 is becoming an increasingly popular target, led by Amgen’s approved T-cell engager Imdelltra, but other groups are looking to improve efficacy. This includes the Chinese company Suzhou Zelgen, which reported promising but early data at ASCO with a new approach, a DLL3 x DLL3 T-cell engager.
The project, alveltamig, produced an overall response rate of 60% across 48 patients in an unconrolled Chinese phase 2 trial in third-line small-cell lung cancer. This looks better than the 40% ORR seen with Imdelltra in the third-line Dellphi-301 trial, which was enough to get that drug FDA accelerated approval in second-line disease.
This led the ASCO discussant, Dr Catherine Meador of Massachusetts General Hospital, to ask: “Are trispecific antibodies truly likely to be more effective than bispecifics? Or is this a function of patient selection or smaller numbers in an earlier phase trial?”
More data could shed light on these questions, she added, although one reason to be cautious is that this number included unconfirmed responses. There are also broader worries that results generated in China might be hard to replicate in other populations.
Two epitopes
The idea behind alveltamig, also known as ZG006, is that binding to two distinct DLL3 epitopes could increase potency over regular T-cell engagers like Imdelltra.
A look at OncologyPipeline shows no other DLL3 x DLL3 T-cell engagers in development, but there are several trispecific approaches in play.
DLL3-targeting bispecifics/trispecifics in clinical development
| Project | Description | Company | Status |
|---|---|---|---|
| Imdelltra | DLL3 TCE | Amgen | AA for 2nd-line SCLC based on 40% ORR in Dellphi-301 |
| Alveltamig (ZG006) | DLL3 x DLL3 TCE | Suzhou Zelgen | China ph2 data in 3rd-line SCLC presented at ASCO 2025: ORR 63% & 58% with 10mg Q2W & 30mg Q2W respectively |
| Obrixtamig (BI 764532) | DLL3 TCE | Boehringer Ingelheim | Ph2 Dareon-5 in 3rd-line SCLC & 2nd-line neuroendocrine tumours, completes Oct 2026 |
| Gocatamig (MK-6070) | DLL3 TCE* | Merck & Co (via Harpoon) | Ph1/2 data in 2nd-line solid tumours at ESMO 2023: ORR 35% across tumour types; ifinatamab-dxd combo trials ongoing |
| Peluntamig (PT217) | DLL3 x CD47 MAb | Phanes Therapeutics | Ph1/2 Skybridge in 2nd-line SCLC & NETs, completes Dec 2027 |
| RO7616789 | DLL3 x 4-1BB TCE | Roche | Ph1 in 2nd-line SCLC & NETs, completed Mar 2025 |
| QLS31904 | DLL3 TCE | Qilu | China ph1 in 2nd-line solid tumours, completed Sep 2024, status unknown |
Note: *also includes human albumin binding domain to extend half-life. Source: OncologyPipeline & clinicaltrials.gov.
Merck & Co’s gocatamig (via Harpoon, previously known as MK-6070) is said to be a “TriTAC”, comprising a targeting moiety, a CD3-binding T-cell engaging domain and an anti-albumin domain. However, the last is merely designed to extend the project’s half-life, so this asset probably can’t be classed as a true trispecific.
Roche’s RO7616789, meanwhile, additionally binds to 4-1BB, a co-stimulatory approach that hasn’t paid off so far. A phase 1 study of this asset ended in March, with no word yet on results.
Roche also has a phase 1 anti-DLL3 ADC, RG6810 (formerly IBI3009) licensed from Innovent in January – another arena in which there are plenty of players, including Zai Lab, whose zocilurtatug pelitecan has impressed on efficacy and is soon set to enter a registrational trial.
Other DLL3-targeting trispecific projects are preclinical: Concept To Medicine Biotech’s DLL3-TOPAbody also hits 4-1BB, while Zymeworks’ ZW209 aims to harness another co-stimulatory domain, CD28 (an IND filing is expected in 2026).
CStone’s CS2010 additionally hits SSTR2, a target made famous by Novartis’s approved radiopharmaceutical Lutathera, used in gastroenteropancreatic neuroendocrine tumours. SCLC is a type of neuroendocrine tumour.
Better than Imdelltra?
For now, Suzhou Zelgen looks set to have the DLL3 x DLL3 niche to itself. The study presented at ASCO tested two doses, 10mg and 30mg every two weeks, both given intravenously. The trial enrolled patients progressed after platinum-based chemo and at least one other systemic therapy; DLL3 expression wasn’t required for enrolment, but was assessed retrospectively.
The ORR was 63% among 24 patients receiving 10mg, and 58% among 24 patients receiving 30mg. According to the swimmers plot, 12 of 15 responses in the 10mg group appear to be confirmed, giving an ORR of 50%. Among 14 responses in the 30mg group, 11 appear to be confirmed (ORR 46%).
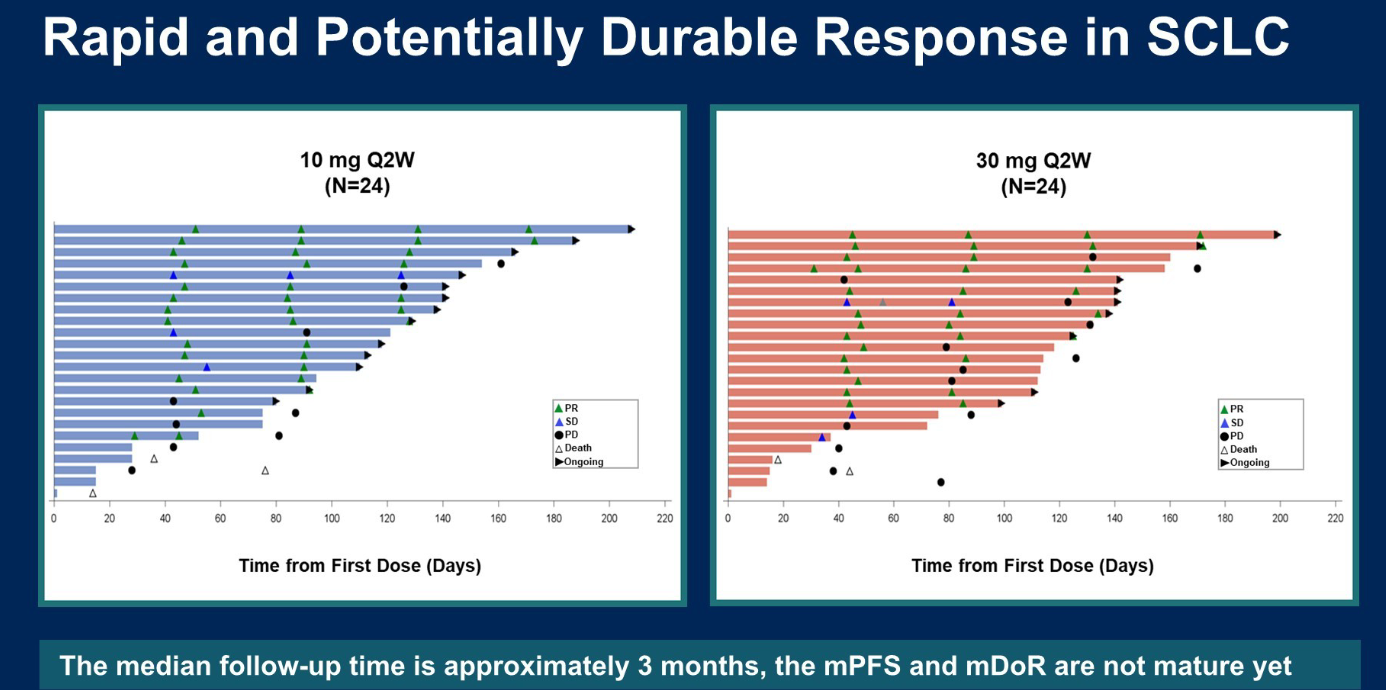
Adverse events looked more troublesome in the higher-dose arm, with higher rates of cytokine release syndrome, including two cases at grade 3 or higher. Meanwhile, there was no grade 3 or higher CRS or ICANS in the 10mg group. There was one death in the 30mg arm that was deemed to be treatment-related, and none in the 10mg arm.
Presenting the data, Dr Xinghao Ai of Shanghai Chest Hospital said final dose selection would depend on more mature data, but 10mg currently seems like the obvious choice. Registrational trials are in the works, she added.
3631













