Innovent pushes its pancreatic hope

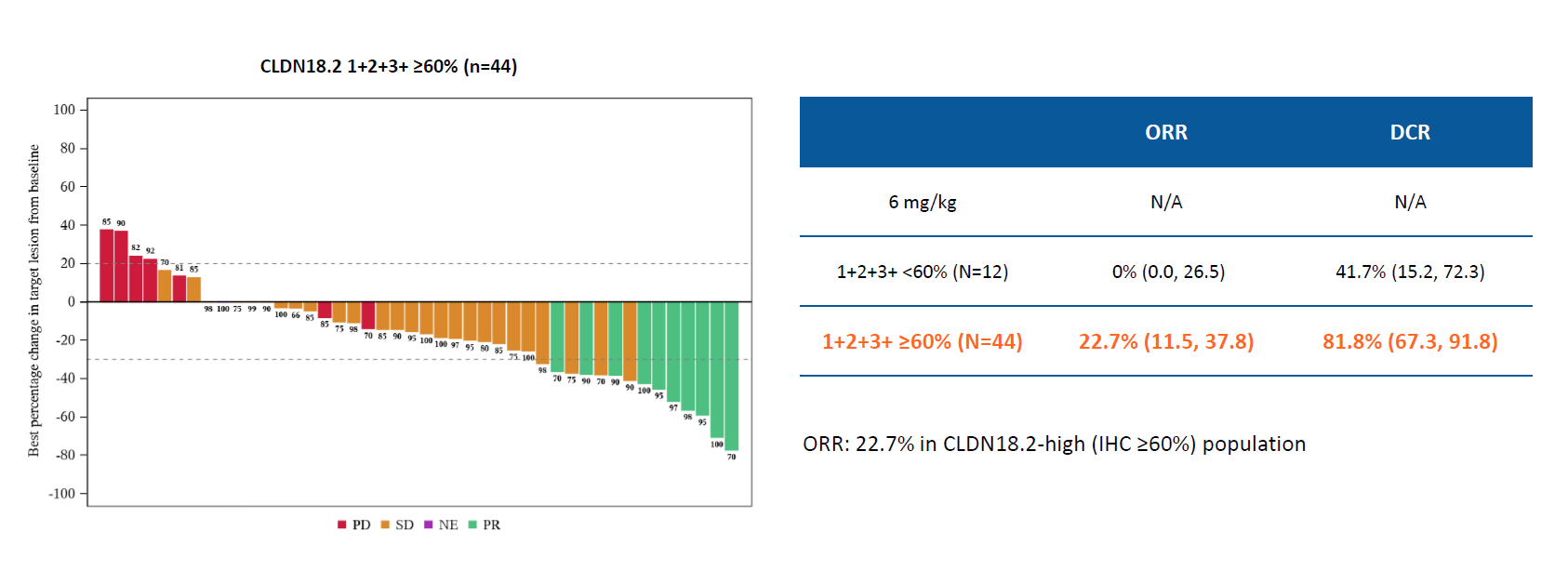
Armed with what Innovent called "remarkable efficacy" in pancreatic cancer, and breakthrough therapy designation from China's NMPA, the company has taken arcotatug tavatecan into phase 3 in this setting. The G-Hope-002 study, just revealed on clinicaltrials.gov, will at the end of this month start to enrol 201 Claudin18.2-positive third-line-plus pancreatic patients, and test arcota-T monotherapy versus placebo. Arcota-T is one of numerous anti-Claudin18.2 ADCs in development across biopharma, but is the only one in phase 3 for pancreatic cancer. In fact, the only other similarly acting molecules in any trials that explicitly include pancreatic cancer are AstraZeneca's AZD4360 and Luszana's garetatug rezetecan, according to OncologyPipeline, Junshi having terminated its phase 1 study of JS107. Innovent's enthusiasm is driven by a multi-tumour basket study that showed an 18% ORR among 56 arcota-T-treated pancreatic cancer patients – a number that went up to 23% considering only the 44 with Claudin18.2 expression of ≥60%. Innovent has highlighted arcota-T's use of an Fc-silenced antibody and site-specific conjugation, and with the phase 3 G-Hope-001 gastric cancer study already under way the ADC is its most advanced oncology project. Still, investors' attention remains on licensing prospects for Innovent's anti-PD-1/α-biased IL-2 fusion protein IBI363.
Arcotatug tavatecan's muti-tumour basket study, advanced pancreatic cancer subgroup
2513