
ASH 2025 – Lilly claims Jaypirca is best
Studies presented at ASH show this to be true only up to a point.
Studies presented at ASH show this to be true only up to a point.

On the eve of ASH Lilly’s BTK inhibitor Jaypirca secured full approval for refractory chronic lymphoblastic leukaemia, in spite of its confirmatory study failing to show an overall survival benefit. Now the company is highlighting earlier lines of CLL therapy, with the Bruin CLL-314 and 313 trials being presented at the conference.
Both studies had been toplined positive, and Lilly is arguing that ‘313, a head-to-head against Imbruvica, shows Jaypirca to be better than the AbbVie/Johnson & Johnson drug. Whether it actually is remains a matter for debate, as does Jaypirca’s precise role in early CLL lines, and the glaring fact that the drug hasn’t been tested against BeOne’s Brukinsa.
Either way, Lilly has consistently argued that the front line isn’t expected to become a dominant setting for Jaypirca. The non-covalent drug was developed for patients who fail on front-line covalent BTK inhibition (Imbruvica, Brukinsa or Calquence), and this setting has only became real with the 3 December US approval, said Jake Van Naarden, president of Lilly’s oncology division.
That’s because Jaypirca’s accelerated nod was for third-line or later CLL, but the full approval, backed by the Bruin CLL-321 trial, specifically relates to second-line, covalent BTK inhibitor-relapsed patients. “The journey of the medicine commercially is only starting now,” Van Naarden told ApexOnco ahead of ASH.
Front-line settings
But if that’s the case, why run ‘314 (a front-line trial against Rituxan plus bendamustine), and ‘313 (Imbruvica head-to-head in first-line and relapsed populations)? “Flexibility,” said Van Naarden.
He sees a cohort of doctors who will simply take the view that Jaypirca is the best drug, and so will want to give it first rather than waiting for a patient to relapse. The results of both trials are being filed with the FDA, which Van Naarden expects to issue Jaypirca with a line-agnostic CLL label similar to Imbruvica’s, Brukinsa’s and Calquence’s.
Current thinking is that Brukinsa is the best covalent BTK inhibitor, since the drug beat Imbruvica on progression-free survival in Alpine, a study in relapsed CLL. But Van Naarden isn’t convinced, claiming that Imbruvica underperformed in Alpine, and pointing to the fact that even AstraZeneca’s Calquence is holding its own in the front line.
Perhaps Lilly’s strongest hand with first-line Jaypirca is the PFS curves, with 0.20 hazard ratio, achieved in Bruin CLL-313 and revealed in an ASH late-breaker on Tuesday. In an analogous BeOne study, Sequoia, Brukinsa beat the same Rituxan/bendamustine control with a hazard ratio for PFS of only 0.42.
Progression-free survival by blinded review in Bruin CLL-313
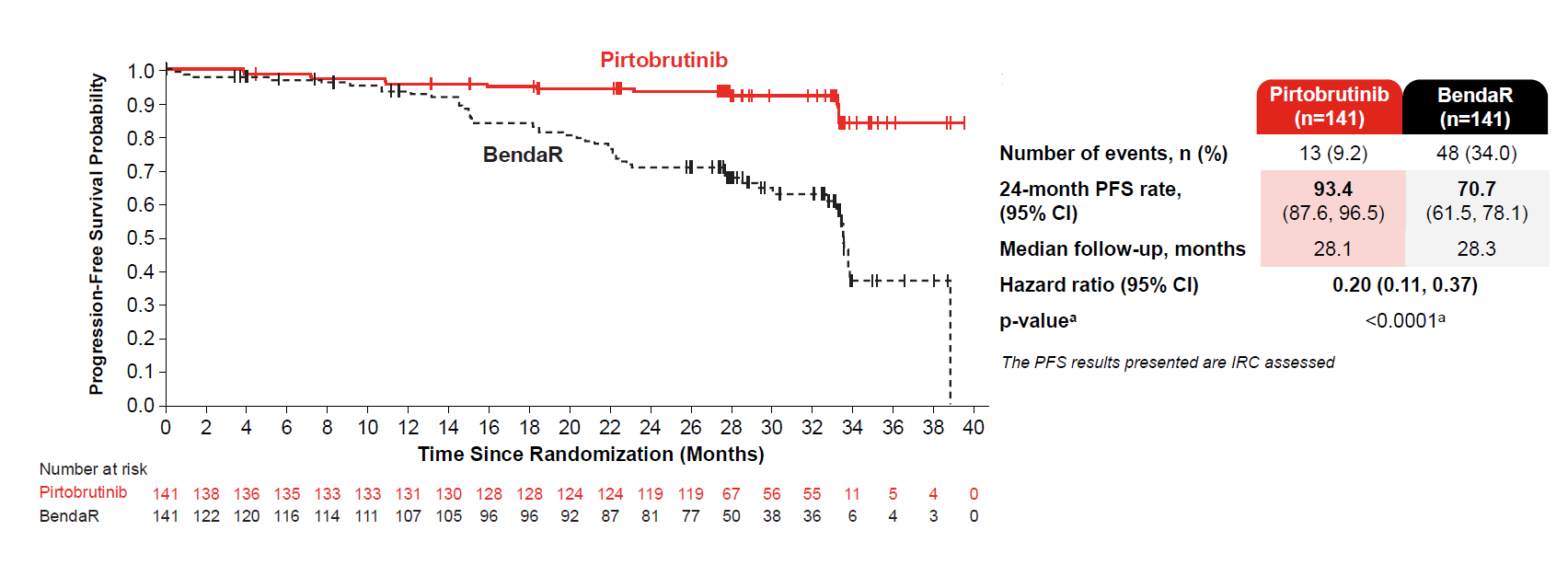
Interpretation of ‘314, meanwhile, is slightly more problematic. That study was designed to show non-inferiority versus Imbruvica on ORR, and this it did, with nominal superiority to boot, at 87% for Jaypirca versus 79% for Imbruvica across both first-line and relapsed populations.
The effect seemed to be driven by relapsed patients, though Lilly argues that this was an artefact of small patient numbers. There was also an important apparent safety advantage, with atrial fibrillation/flutter seen in just 2% of Jaypirca patients, versus 14% of those on Imbruvica.
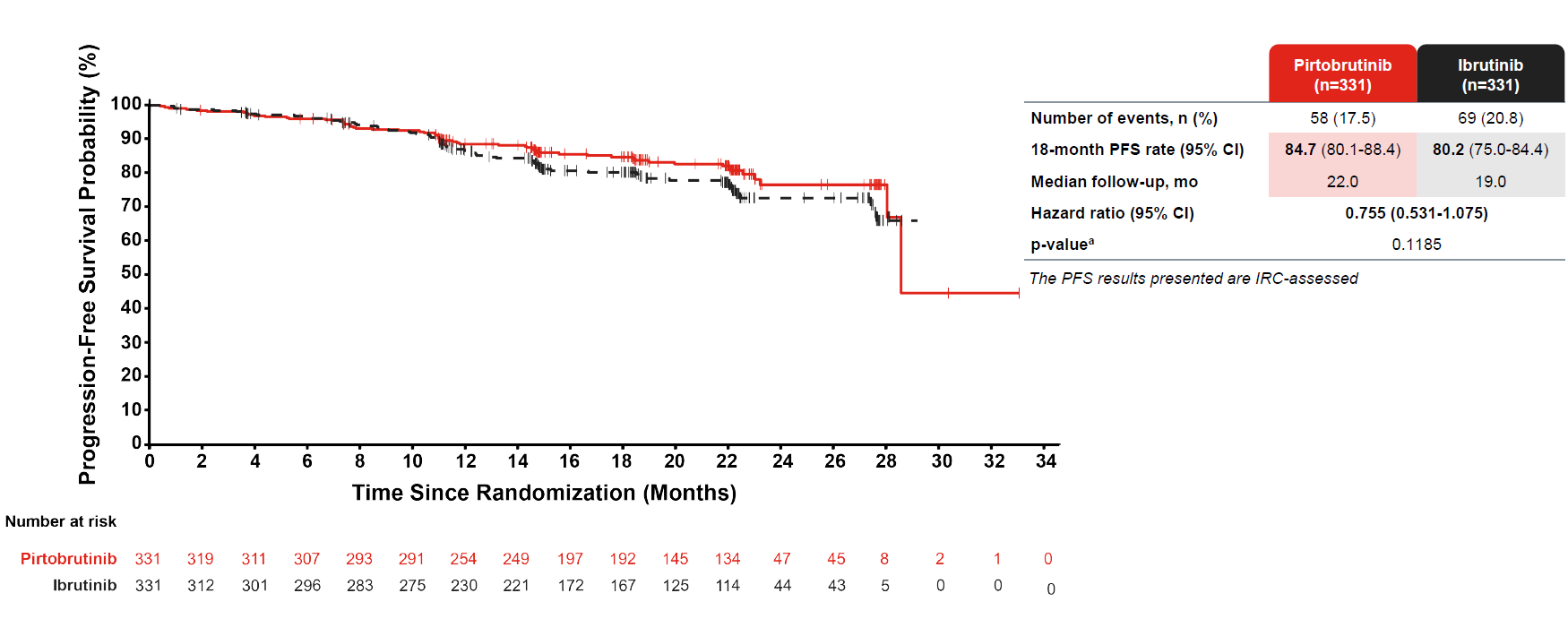
The problem comes with PFS, for which a secondary endpoint called for superiority by blinded review. In July Lilly claimed that this showed a trend in favour of Jaypirca, but an ASH presentation on Monday revealed this only to be true for investigator-assessed PFS, while curves by blinded review showed minimal separation, and a nominal p value of 0.1185.
David Hyman, chief medical officer of Lilly’s Loxo division, said the blinded PFS endpoint was chosen for regulatory reasons, claiming: “What practising physicians care about is what they experience, which is investigator-assessed PFS.”
Meanwhile, Van Naarden also said ‘314’s relapsed cohort showed Imbruvica to be outperforming the controversial Alpine study where Brukinsa showed superiority. But direct comparison is impossible since Alpine PFS numbers are by blinded review, while those split out from ‘314 are by investigator assessment.
The war of words between Lilly and BeOne is set to continue, with Van Naarden rejecting a suggestion that Jaypirca will be squeezed by Brukinsa in the front line, and by BeOne’s BTK degrader BGB-16673 on the other. “I haven’t seen a clinical dataset from the degraders that suggest to me that [BeOne’s Jaypirca head-to-head] study will be successful,” he said.
Before that Jaypirca has a chance to make a mark in its intended setting, while the front line remains in the hands of regulators.
Progression-free survival by blinded review in Bruin CLL-314
1108













