An ozekibart reminder about liver toxicity
Inhibrx reveals three deaths in phase 1, but all predate screening protocols.
Inhibrx reveals three deaths in phase 1, but all predate screening protocols.

As one of the few inhibitors of DR5 still in development, Inhibrx’s ozekibart piqued market interest when its pivotal phase 2 Chondragon trial was said to have hit on progression-free survival last month. A measure of the enthusiasm is that since then Inhibrx shares have nearly tripled.
Investors might therefore have winced at a reminder of problems that had plagued ozekibart’s early development, with the quiet disclosure on Thursday of summary results from its phase 1 study, which detailed three ozekibart-related deaths from liver toxicity. However, all predate implementation of a special screening protocol, and the company says none has occurred since.
The phase 1 data were presented at the Industry/Academia Precision Oncology & Radmed Symposium, but relate to an old cutoff of 2 August 2024. Notably, in 2023 ozekibart was put on clinical hold after Inhibrx was told to add screening for the Hepatic Steatosis Index into enrolment protocols, and the data spell out why this was necessary.
Not a problem
“We don’t consider safety to be a problem,” Inhibrx’s chief financial officer, Kelly Deck, told ApexOnco. “Once we put screening, monitoring and remediation protocols in place there have been no grade 5 liver tox events, and any patients with high-grade elevations resolved quickly following protocol.”
Meanwhile, full phase 2 Chondragon results are to be unveiled on 14 November at the Connective Tissue Oncology Society meeting, so for now little is known about toxicity there. Last month the company said Chondragon, a chondrosarcoma study, had met its primary endpoint, yielding median PFS of 5.5 months versus 2.7 months for placebo (hazard ratio of 0.479, p<0.0001).
Inhibrx has said nothing yet about overall survival in this trial, which allowed placebo recipients to cross over to ozekibart on progression. The group aims to file ozekibart on the back of the Chondragon data in the second quarter of next year.
Deck also confirmed that there was one death from liver toxicity in Chondragon, but this too predated the new screening protocol.
As for indications beyond chondrosarcoma, Inhibrx’s phase 1 data summary related to Ewing sarcoma and colorectal adenocarcinoma. The former appears promising, with ozekibart monotherapy prompting a 54% best response rate among 13 patients given monotherapy, and 64% among 25 given a combo with irinotecan and Temodar.
Inhibrx cited ORRs of 15-30% in separate trials of irinotecan/Temodar alone, but the ozekibart result includes unconfirmed responses. In colorectal adenocarcinoma the monotherapy best response rate is 31% (13 patients), and 23% among 26 given an ozekibart/Folfiri combo.
Confusion
The multi-tumour phase 1 trial last yielded data from its colorectal adenocarcinoma cohort, at January’s ASCO-GI symposium, where there was one death, from neutropenic sepsis. An earlier presentation, from a chondrosarcoma cohort at last year’s Connective Tissue Oncology Society meeting, revealed two deaths from liver failure.
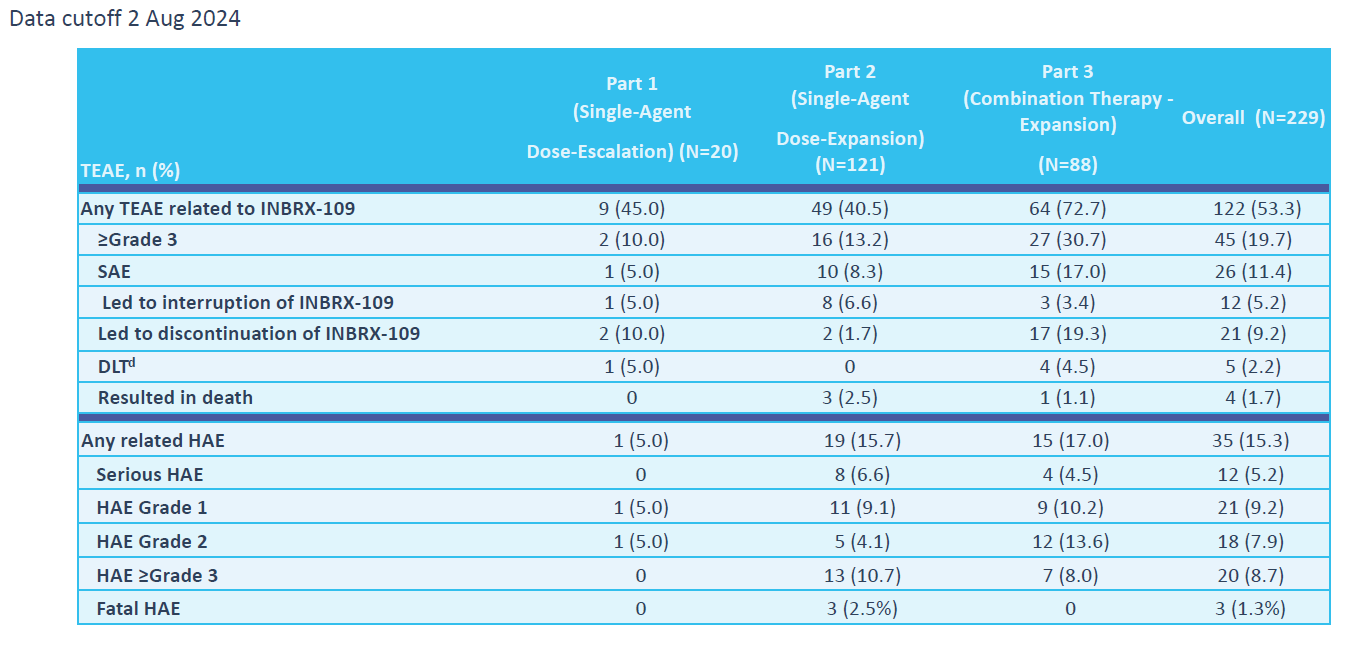
As such, the new summary data add an extra death from hepatotoxicity, and all were said to be related to ozekibart. Overall there were 229 patients in this study, across monotherapy and combination cohorts, for a liver-related fatality rate of 1.3%. The rate of grade 3 or higher liver toxicity across this trial stands at 8.7%, but again this will include the pre-screening period.
“In hindsight, the data should never have been presented in such a manner because all it did was cause a lot of confusion,” Deck said.
Ozekibart's phase 1 safety summary
After this story was published Inhibrx pointed out that the additional death occurred in a mesotheliooma patient in 2020, and had been disclosed in the company's IPO document.
2553













