
ASCO 2025 – zilo-V adds a patient death to its tox tally
The second line shows no respite for Merck & Co's ROR1-targeting ADC.
The second line shows no respite for Merck & Co's ROR1-targeting ADC.

A relapsed/refractory lymphoma study of Merck & Co's zilovertamab vedotin has shown similar toxicity concerns to those seen in the first-line setting. True, the worst adverse events again came at a dose slightly higher than that being taken forward in phase 3, but there's now a patient death to contend with, ASCO heard on Friday.
As for efficacy, a headline response rate of 56% was seen among 16 subjects given the go-forward 1.75mg/kg zilo-V dose. While that's better than historical numbers, the ASCO discussant noted more impressive efficacy on a cross-trial basis for other novel combos, including those of Roche's Columvi and Genmab's Epkinly, in a similar setting.
The phase 2/3 study presented at ASCO is Waveline-003, combining zilo-V with Rituxan/gemox chemo. The data concerned an uncontrolled dose-confirmation stage, but the trial's phase 3 portion is now now enrolling 200 patients across two cohorts, zilo-V plus R-gemox and a comparator of R-gemox alone.
Presenting the results, Dr Philippe Armand of Dana Farber said zilo-V efficacy was similar for 1.75mg and 2.0mg/kg doses, with ORRs of 56% and 57% in 16 and seven patients respectively.
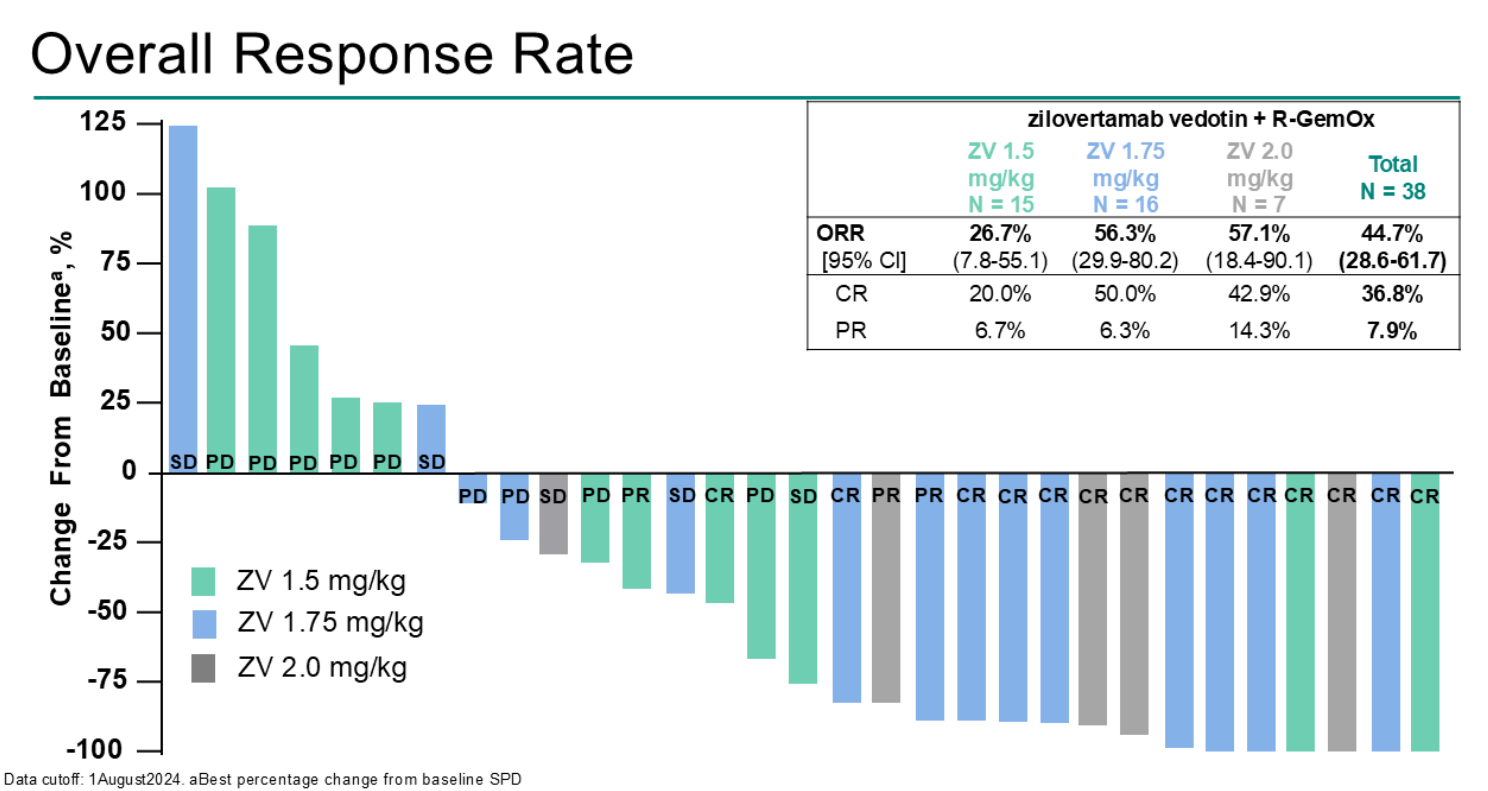
However, all 16 patients in the go-forward dose group experienced drug-related adverse events, including five rated serious and 10 at grades 3 to 4. Even worse was the only slightly higher 2.0mg/kg dose, where two subjects discontinued owing to sepsis or respiratory fatigue, and a third patient died of sepsis.
In the first-line phase 2 Waveline-007 study, presented at last year's ASH, there were similar concerns over zilo-V doses of 2.0mg/kg or above; 1.75mg/kg itself had a profile suggesting that Merck was treading a fine line in seeking a therapeutic window. However, there were no zilo-V-related deaths.
Zilo-V is an anti-ROR1 ADC, and first-line lymphoma is its other phase 3 setting, in the Waveline-010 trial.
Old and new comparators
Armand argued that response rates in Waveline-003 compared well against historical numbers for R-gemox. This is true to an extent for ORR (40-50% for R-gemox), and especially so for rates of complete remission: about 30% in the historical data, against 50% for zilo-V's 1.75mg/kg dose.
However, discussing the results, Cleveland Clinic's Dr Paolo Caimi pointed to recent trials of R-gemox combos of Columvi and Epkinly in relapsed lymphoma. The former showed ORR and CR rates of 68% and 59% respectively in the Starglo study, while the latter yielded 85% and 61% in Epcore NHL-2.
What's more, Caimi said Waveline-003 patients appeared not to have been heavily pretreated, having received a median of two prior therapy lines, and only two of 40 having had prior Polivy. In summary, Caimi said Merck's data were "not (yet) practice changing".
Such issues are relevant in light of the $2.8bn that Merck paid to acquire VelosBio, zilo-V's originator. The project is still in play, but it risks being overtaken by others, as well as having a seemingly narrow therapeutic window.
1796













