
ASH 2023 – sense and serendipity in Dizal's Jackpot win
Dizal got a lucky break with golidocitinib, and now mid-stage data back the project’s activity in T-cell lymphoma.
Dizal got a lucky break with golidocitinib, and now mid-stage data back the project’s activity in T-cell lymphoma.

Almost by accident Dizal discovered that focusing on one subtype of the JAK protein, specifically JAK1, might play a role in T-cell lymphoma, and now this work has resulted in a project that could soon see its first approval.
Golidocitinib, the asset in question, has yielded a 44% confirmed overall response rate in the phase 2 part of its Jackpot-8 trial, the ASH conference heard yesterday, and the most positive scenario has this serving as the basis for an accelerated US approval. That’s quite a turnaround for a project whose original development plan was entirely different.
Golidocitinib was actually designed to work in lung cancer, based on reports that the JAK/STAT pathway might play a role in Iressa resistance, Dizal’s chief executive, Xiaolin Zhang, told ApexOnco. “But when we screened our panels we found that cell lines sensitive to golidocitinib all shared a T-cell origin.”
“With that finding we collected patient samples to look whether the JAK/STAT pathway was active in peripheral T-cell lymphoma (PTCL),” said Zhang. “Yes it was.” Thus development turned to PTCL, and the Jackpot-8 data offer the most robust backing for this approach so far.
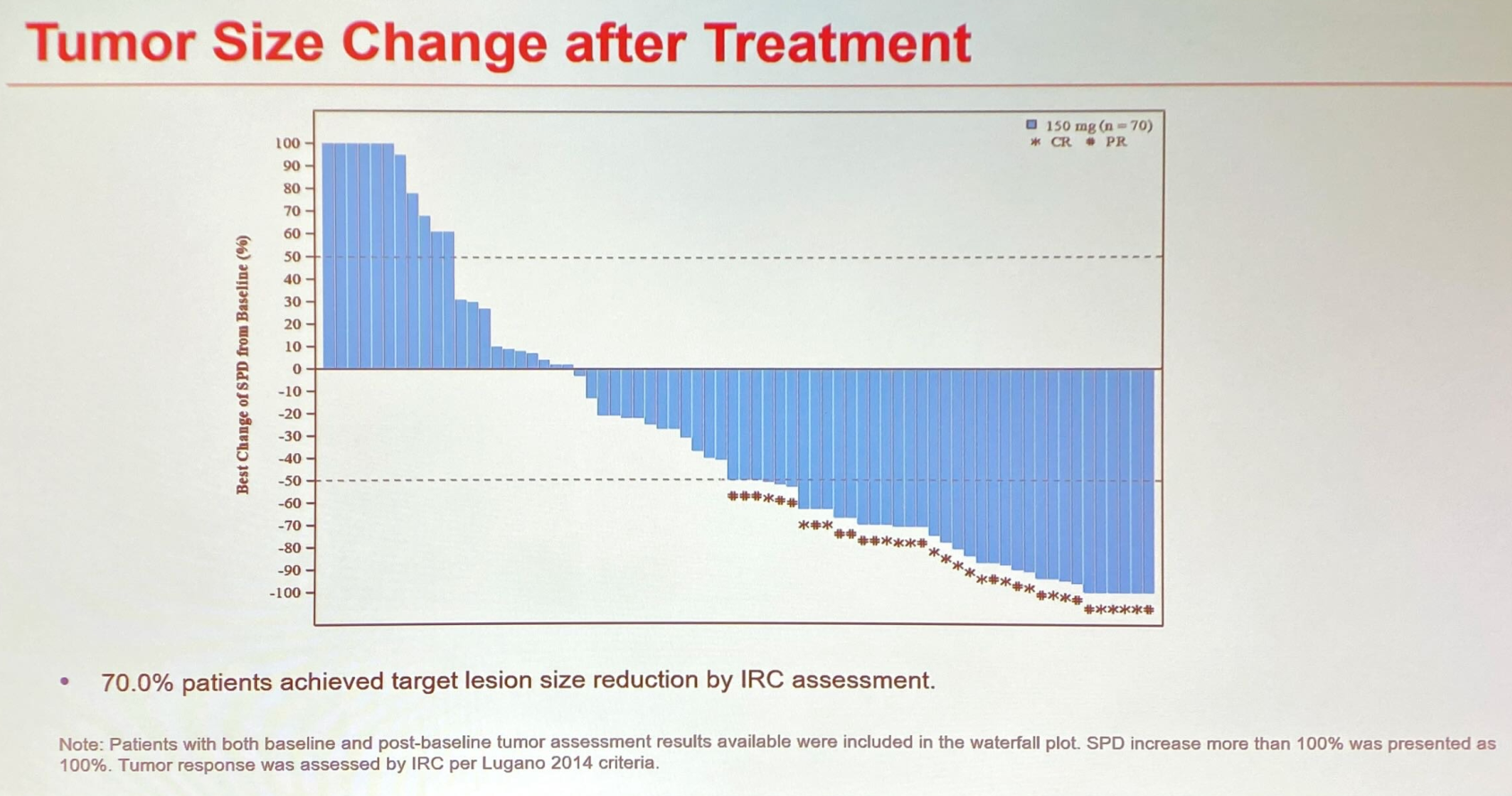
Jackpot
PTCL is an aggressive but relatively rare cancer in which HDAC inhibitors such as Beleodaq have been used without great success. But there is no standard treatment, and Dizal cites a five-year survival rate for patients of under 30%.
In Jackpot-8 the 104 enrolled subjects had failed a median two prior therapies, all being post-chemo and most having also had an HDAC inhibitor. Against such characteristics an ORR of 44% in 88 evaluable patients, including a 24% rate of complete remissions, looks impressive. The data also look similar to those seen with EZH1/2 inhibitors in PTCL, presented separately at ASH on Saturday.
One patient-related death was graded treatment related, and while the presenter yesterday cited liver dysfunction as the reason Zhang said this was a mistake, the actual cause being pneumonitis. Either way, golidocitinib has been dosed higher than the 150mg of Jackpot-8 with good tolerability, Zhang insisted.
Based on the data golidocitinib is awaiting approval in China, while in the US it has fast-track designation, and Dizal is at present mulling a regulatory plan. Jackpot-8 includes US hospitals in addition to many Chinese, South Korean and Australian centres, though it has no European involvement.
It was designed as a single-arm study with accelerated approval in mind, but whether this alone will suffice has yet to be determined. The FDA is becoming cautious, and wants a confirmatory study to be substantially under way as a condition of granting accelerated approval. Dizal is discussing the design and scope of a “truly global” trial that would fit the bill.
Efficacy in Jackpot-8
As for golidocitinib’s discovery, “it’s a very scientific story”, said the chief exec. It’s tough to design a truly JAK1-specific molecule because the JAK subclasses are structurally very similar, and Dizal claims that golidocitinib has over 200-fold selectivity for JAK1 over the other JAK proteins JAK2, JAK3 and TYK2.
Interestingly, the molecule carries the code AZD4205, but Zhang said this was only because of Dizal’s origin as a spin-out of AstraZeneca’s Shanghai-based Asian R&D centre. Astra has no direct rights over golidocitinib, but retains a 25% equity stake in Dizal, which floated on the Shanghai stock exchange in late 2021.
Targeting JAK is mechanistically associated with projects for autoimmune disease, while Incyte’s Jakafi, for instance, is established in myelofibrosis. But Jakafi hits JAK1 and JAK2, and CTI Biopharma’s Vonjo, for instance, is touted as a JAK2-specific drug that doesn’t inhibit JAK1.
The only other JAK1-specific molecules to have been in development, according to OncologyPipeline, are Incyte’s itacitinib and Jiangsu HengRui’s SHR0302. The former failed in the phase 3 Gravitas-301 study in graft-versus-host disease, and remains in early trials for treating cytokine release syndrome.
The latter is more interesting competitively, as it recently started a phase 1 trial in peripheral T/NK-cell lymphoma. This seems to be a study at a single hospital in China, and has a primary completion date of November 2025.
1374













