SITC 2025 – fresh hope for Summit's Harmoni
Harmoni-A hits, backing a quiet disclosure about the Harmoni trial.
Harmoni-A hits, backing a quiet disclosure about the Harmoni trial.
It’s all very well for Akeso’s Chinese lung cancer study of ivonescimab, Harmoni-A, to confirm its positive overall survival hit at final analysis, as it did over the weekend at SITC, but market attention lies elsewhere.
That’s because ivonescimab is already approved in China on the basis of Harmoni-A, in EGFR-mutant NSCLC after progression on anti-EGFR TKI therapy. For investors in Akeso’s partner Summit it's more important that the Harmoni-A survival curves back an earlier detail dribbled out concerning the analogous, but global, Harmoni trial, which includes many patients from Harmoni-A, but which failed for OS.
The detail was that a recent analysis of the overall survival curves in Harmoni had shown a slightly improved hazard ratio, with a confidence interval upper bound now at 0.98, rather than the 1.01 when final analysis was triggered. This suggests that, while the median OS numbers remain where they are, a long survival “tail” is helping to push Harmoni into nominally positive territory.
This is all relevant because when Summit first tried to file on Harmoni in the US, backed by a PFS benefit but a negative OS result, it was rebuffed by the FDA, which advised it that a regulatory submission couldn’t be made without a statistically significant OS result. Recently Summit revealed that it was pushing ahead with a US filing anyway.
September read
The trigger for this was an OS analysis of Harmoni done in September, four months after Harmoni’s final OS analysis showed no statistical benefit, and a 0.79 hazard ratio for risk of death (p=0.057).
The subsequent analysis resulted in a slightly improved 0.78 hazard ratio, and a nominal p value of 0.0332, Summit says. The nominal p value matters less than the fact that the confidence interval upper bound is now below 1.00, a fact that suggests no increased risk for patients in the active arm, given ivonescimab plus chemo, versus chemo alone.
Investors will also be relieved by Summit’s claim that the OS result was consistent between Hamoni’s western (median OS 17.0 months versus 14.0 months, HR=0.84) and Asian (median OS 16.7 months versus 14.0 months, HR=0.76) patients. It was the fact that earlier Harmoni data suggested a geographical divergence that undermined Summit’s plans to use the trial to back US approval.
Harmoni is analogous to Akeso’s Chinese Harmoni-A trial, except that it mandates prior failure specifically on third-generation EGFR inhibitors like Tagrisso. The FDA allowed Summit’s trial to pull in those patients from Akeso’s Harmoni-A who met this criterion, and these make up around two thirds of the Harmoni population.
Harmoni-A final
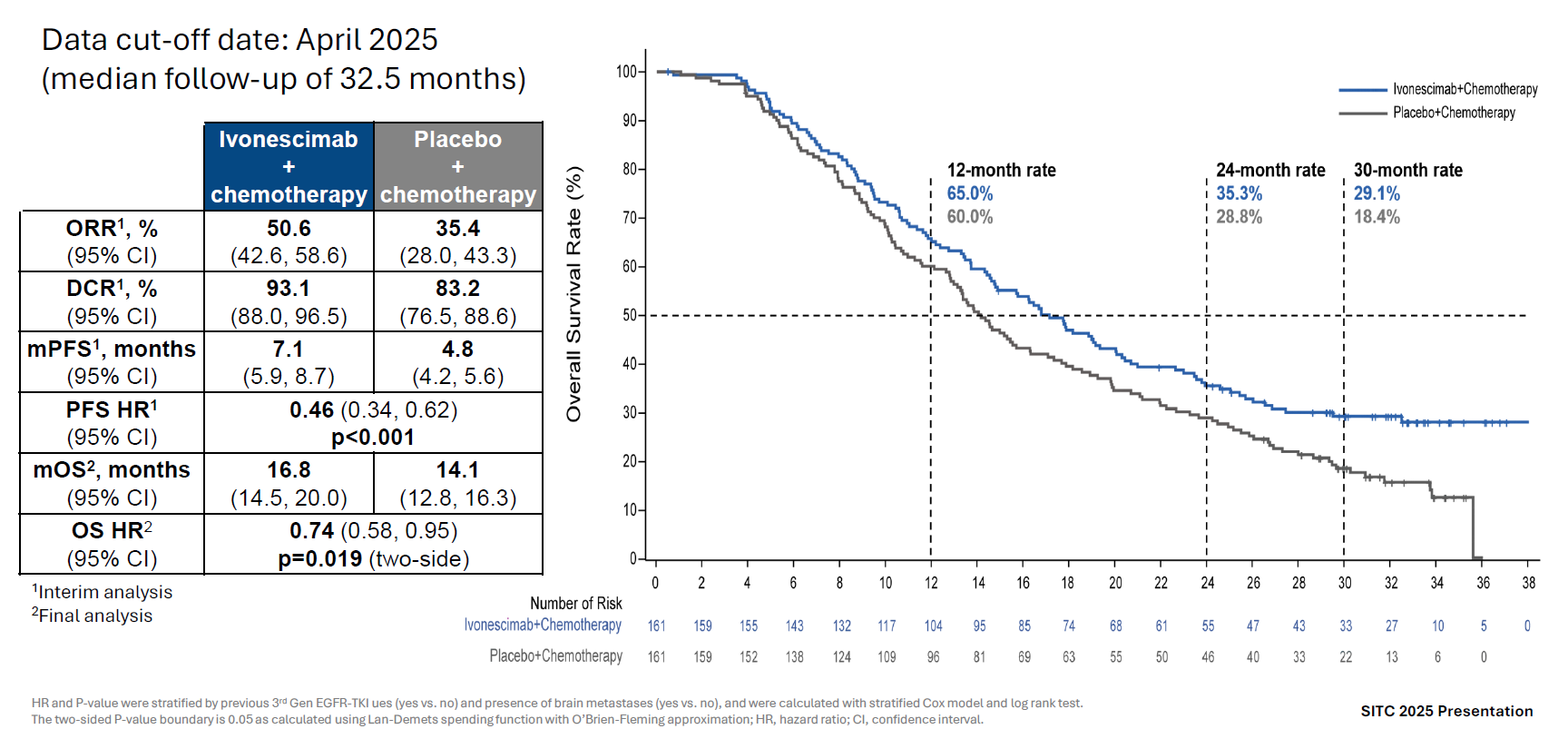
This is why the result of one trial is relevant to the other, and indeed final OS analysis from Harmoni-A, along with the final survival curves, does suggest that a survival tail is helping improve the risk of death across the entire Harmoni and Harmoni-A studies.
Specifically, the final OS from Akeso’s Harmoni-A has yielded medians of 16.8 months for ivonescimab plus chemo, versus 14.1 months for chemo alone, and a 0.74 hazard ratio (p=0.019). Harmoni-A was toplined as positive for OS in August, but the final numbers show an improvement in the hazard ratio, which had deteriorated from 0.72 at first to 0.80 at second analysis.
In the grand scheme, of course, fist-line NSCLC settings, like that of the recently upsized Harmoni-3 trial, are becoming increasingly important for Summit. But the new disclosures around Harmoni and Harmoni-A could increase the chances of ivonescimab’s US approvability.
Final analysis of OS from Akeso’s Harmoni-A trial
3395













