
ASH 2025 – Cullinan finds its new purpose in FLT3

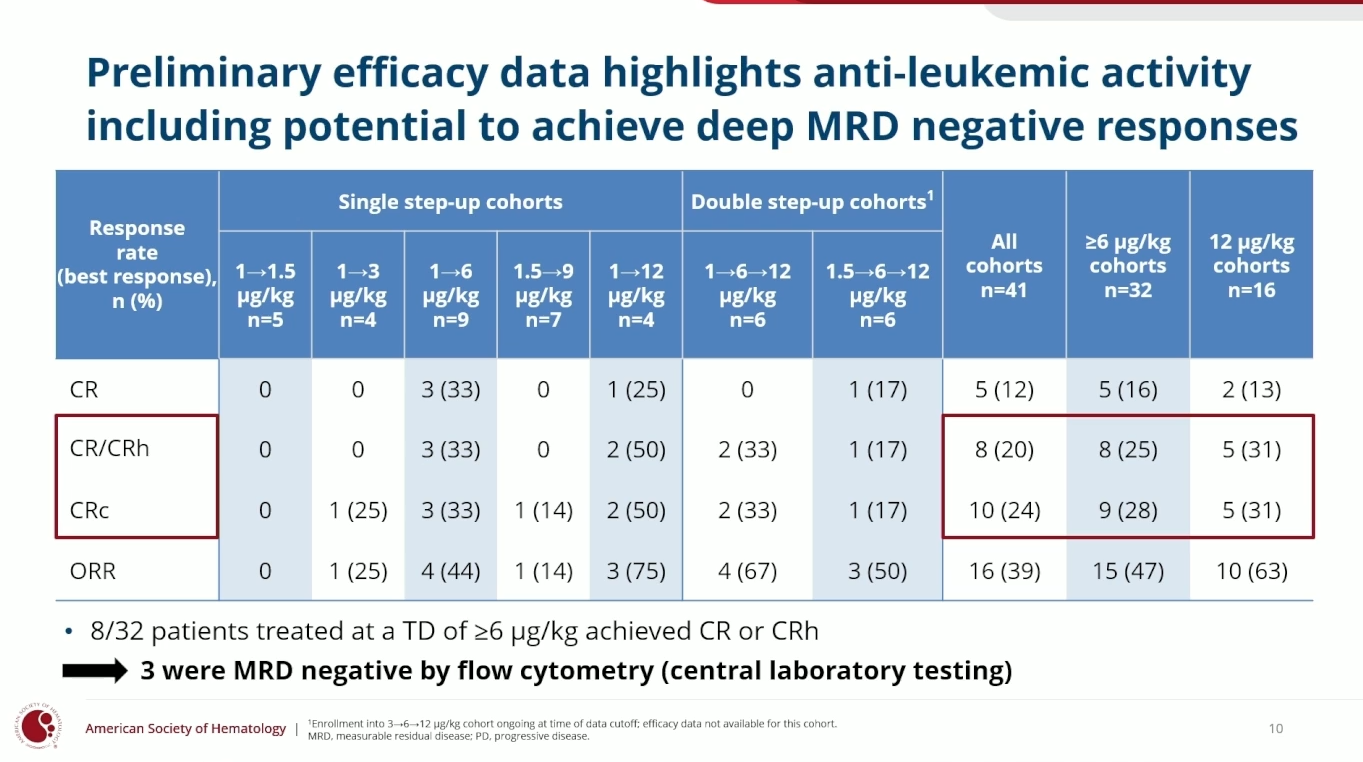
Cullinan, a company seeking fresh purpose since selling ex-US rights to the EGFR inhibitor zipalertinib back to Otsuka, might have found one in CLN-049. This FLT3-targeting T-cell engager’s potential in relapsed/refractory AML and myelodysplastic syndromes was revealed at ASH on Monday, sending the company’s shares up 17%. The efficacy highlight was a 20% rate of complete remission/CRh (CR with partial haematologic recovery) among all dosing cohorts, rising to 31% among 16 patients treated at the highest, 12µg/kg. The company earlier said CLN-049 might be eligible for accelerated approval in relapsed AML with a single-arm study in 80-100 patients, if this could replicate a 20-30% CR/CRh rate, and aims to start one once it settles on the best dose. First-line AML is to be pursued in parallel, with a phase 1/2 study starting next year combining CLN-049 with standard of care. Astellas’s FLT3 inhibitor Xospata is approved for AML with a FLT3 mutation, but CLN-049 being antibody-based means it hits wild-type as well as mutated FLT3; interestingly, in the ASH study there was no correlation between responses and baseline FLT3 expression. OncologyPipeline reveals no other anti-FLT3 T-cell engagers in clinical development, Amgen having discontinued emirodatamab in 2023.
1634













