
World Lung 2025 – Candel looks to a new agla-vec use
But results come from a curated dataset, with no in-trial comparator.
But results come from a curated dataset, with no in-trial comparator.

While primarily targeting its viral immunotherapy aglatimagene besadenovec at localised prostate cancer, Candel Therapeutics is setting its sights on another possible indication: non-small cell lung cancer patients with inadequate response to checkpoint blockade.
Hopes for this latter use might be stoked by phase 2 data presented at a World Conference on Lung Cancer late-breaker on Tuesday, where the bold claim was made that agla-vec might improve survival after injection into "only one or two tumours". Still, the comparison is against historical controls, and the data come from the per-protocol analysis of a trial that disappointed earlier.
Agla-vec is a replication-defective adenovirus encoding the HSV-thymidine kinase gene, administered with valacyclovir, and is basking in its phase 3 win in localised prostate cancer. This helped Candel raise $80m from investors last December, staving off this year's feared financing crunch, and giving the group cash to last into the first quarter of 2027.
But an uncontrolled phase 2 NSCLC trial gave an unexpected extra boost when in May it was said to have "prolonged" median OS in patients with baseline progressive disease despite immune checkpoint therapy. Candel cited median OS of 21.5 months in 41 patients (a separate cohort comprised subjects with stable disease).
It's these data that have just been presented at World Lung, where University of Pennsylvania's Dr Charu Aggarwal unveiled the survival curve for this cohort, citing as comparison historical median OS of 9.8-11.8 months for docetaxel in this type of PD-(L)1-refractory population. In Candel's study patients got two agla-vec injections on top of continued PD-(L)1 blockade, with or without chemo.
She also cited an abscopal effect – implying shrinkage of tumours that weren't directly treated. 35 patients presenting with multiple tumours didn't have agla-vec administered to all of these, yet two thirds experienced regression of their non-injected tumours, she claimed.
OS curve for post-checkpoint NSCLC patients with baseline progressive disease
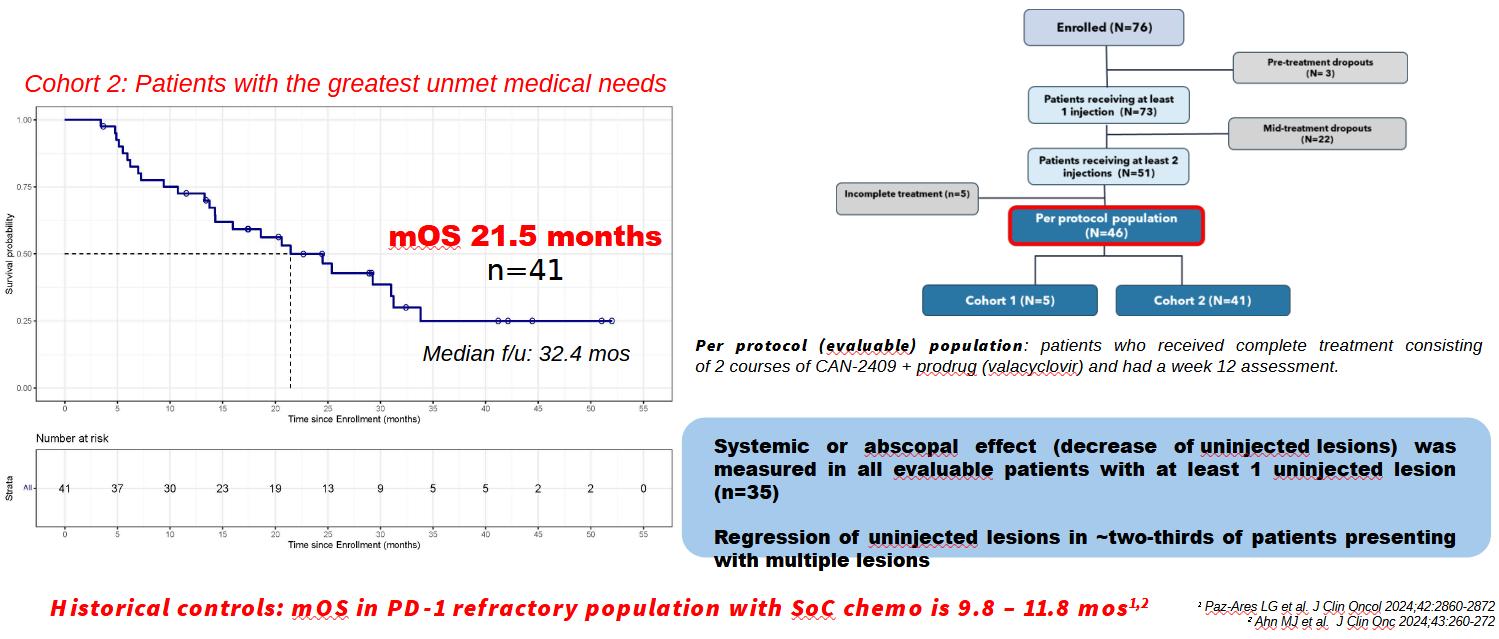
Such boasts will of course need substantiating against a true comparator, and this study's uncontrolled setting is a drawback. Another red flag is that in presenting the data Candel has zeroed in on just over half the patients who were actually enrolled into this trial.
The sharp-eyed will note that 25 subjects dropped out, most after receiving only one agla-vec injection, and most because of disease progression; this will likely have preselected for the fittest patients, who would naturally have been expected to live longest. No intent-to-treat survival numbers or curves were shown at World Lung.
Neither were response rates disclosed; that's relevant since this same study disappointed at ASCO 2024 when a poster revealed an ORR of just 11% among 45 patients. True, with survival data available ORR becomes less important, and it's possible for some patients to derive a survival benefit while in stable disease, and thus not formally being classed as responders.
After its recent cash raise Candel had $101m in the bank at the half-year point, and naturally the localised prostate cancer indication remains the primary focus for agla-vec. Given that the phase 3 win in this setting came nine months ago it remains a puzzling fact that Candel doesn't expect to file in the US until the fourth quarter of 2026.
Candel has admitted making "minor" changes to the prostate cancer trial's design without obtaining an SPA (special protocol assessment) amendment. More recently it said only "certain data generated from this study could be sufficient" for approval, so whether the SPA still applies to the phase 3 study in its current form remains another key consideration.
After this story was published Candel told ApexOnco that it had obtained written confirmation from the FDA that the SPA was still intact after the minor protocol amendment.
1267













