
World Lung 2025 – Summit's Harmoni guessing game
Investors now have more reasons to doubt Harmoni's ability to back US approval.
Investors now have more reasons to doubt Harmoni's ability to back US approval.

The high-profile Harmoni trial of ivonescimab, toplined by Summit to market disappointment in May, looks to have disappointed again. Full data, unveiled at a plenary session of the World Conference on Lung Cancer on Sunday, have revealed a disparity in progression-free survival between Asian and western patients, casting further doubt on ivonescimab's ability to impress beyond China.
The data also confirmed Harmoni's failure to show an overall survival benefit at final analysis, a fact that calls into question Summit's ability to file ivonescimab in the US. The FDA earlier told the company that a regulatory submission couldn't be made without a statistically significant OS result.
That Harmoni data show a geographical divergence undermines hope that Akeso's Chinese Harmoni-A trial might have read across positively to Harmoni. The former trial's patients make up two thirds of the Harmoni population. Akeso, ivonescimab's originator, recently said Harmoni-A had shown a statistically significant and clinically meaningful OS benefit.
The fact that Asian patients appear to be doing better than western subjects suggests that Harmoni might never show an OS benefit, even if Harmoni-A does. This is especially pertinent given that survival numbers are deteriorating over time – something already seen with OS in Harmoni-A, and now revealed to be the case with PFS in Harmoni.
Curves revealed
Harmoni compared ivonescimab plus chemo against chemo alone in patients with non-squamous EGFR-mutant lung cancer who progressed on a third-generation EGFR inhibitor like Tagrisso. In May Summit revealed Harmoni's hazard ratios: 0.52 (p<0.00001) for PFS, and 0.79 (p=0.057) for OS.
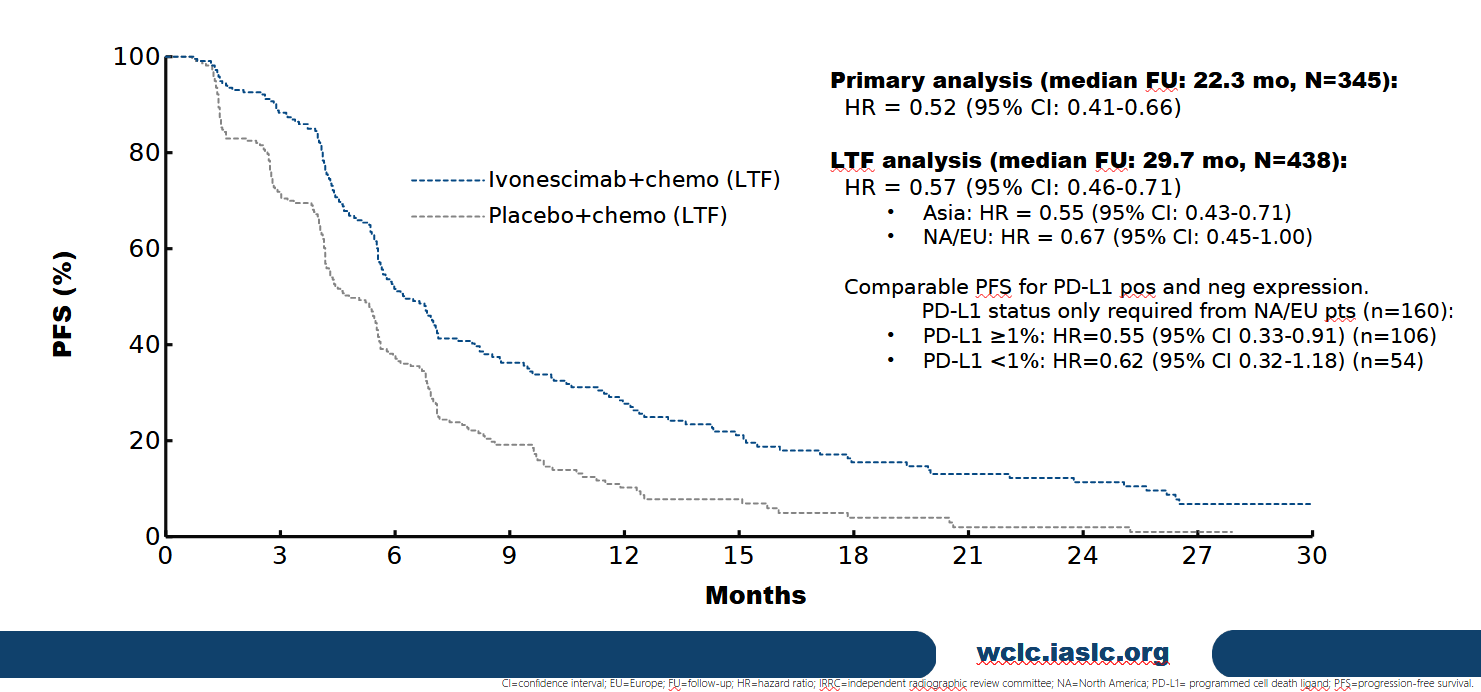
World Lung showed the survival curves for the first time, revealing the absolute benefits across the entire study. These showed median PFS of 6.8 months versus 4.4 months for control at final analysis, which took place with median follow-up of 22.3 months.
So far so promising, but a separately disclosed longer-term follow-up of PFS, at 29.7 months, will cause nervousness among Summit investors, who sent Summit stock down 15% on Monday. This subsequent read yielded a PFS hazard ratio of 0.57, implying a worsening from the 0.52 reported at "final" PFS analysis.
And it's the longer-term PFS follow-up that also reveals Harmoni's geographical disparity. The PFS hazard ratio for Asian patients came in at 0.55, but that for North American/European subjects was a far less impressive 0.67.
PFS in Harmoni with long-term follow-up
There has been much guesswork by investors and analysts in using Akeso's disclosures of Harmoni-A to try to extrapolate Harmoni results. The two studies are closely linked because the FDA allowed Summit's trial to include all Harmoni-A patients who had received a third-generation kinase inhibitor; this appears to have amounted to 273 patients – 62% of Harmoni's 438-strong population.
As such, despite Harmoni disappointing in May, hopes had been stoked by Summit stating that the trial's PFS benefit was consistent between Asian and western patients, and by Akeso's subsequent revelation that Harmoni-A yielded a positive OS result. Full numbers from Harmoni suggest that the first claim hasn't played out with longer-term PFS follow-up.
As for OS in Harmoni, it was perhaps not entirely appreciated that when Summit toplined the hazard ratio this related to a final – and not interim – analysis. As such, the only way the OS numbers can now improve is if a sufficiently large "tail" of long-term survivors develops, lowering patients' risk of death across the entire study.
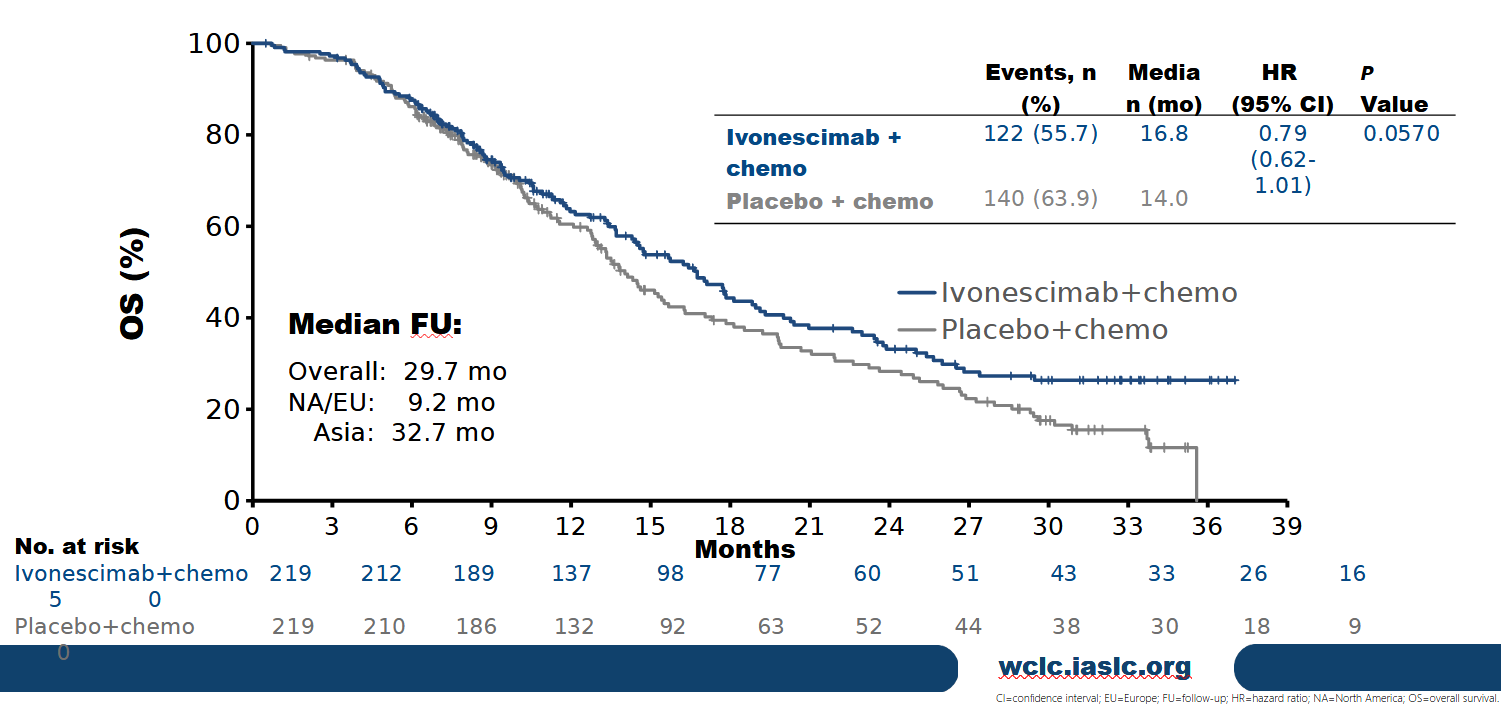
Final OS curves, presented at World Lung, show no difference until nine months, with meaningful separation not seen until considerably later. Though the issue has now entered the realms of minutiae, the World Lung presenter, Dr Jonathan Goldman of UCLA, said that Harmoni's North America/Europe OS follow-up was "not yet mature", even though follow-up across the entire trial population clearly was.
This might support the belief that the curves could separate further, and indeed Goldman unveiled more mature OS curves, relating to a September cutoff for non-Asia patients, where the hazard ratio was a marginally better 0.78, and its confidence interval upper bound crept just below 1.00, to 0.98. Whether this, or any further flattery courtesy of a survival tail, makes a difference will be up to the FDA.
Overall survival curves at final analysis of Harmoni
This story has been updated to include longer-term survival data.
3675













