
Adicet pivots
After disappointments in aggressive lymphomas Adicet focuses ADI-001 development on mantle cell lymphoma and goes into lupus.
After disappointments in aggressive lymphomas Adicet focuses ADI-001 development on mantle cell lymphoma and goes into lupus.

Various updates from Adicet have caused confidence in the group’s initially promising off-the-shelf gamma-delta Car-T therapies to ebb away, and today the pivot came: Adicet is scaling back work with its lead asset, ADI-001, in lymphoma, and launching a new programme in lupus.
Looking to autoimmune diseases like lupus follows a recent trend among Car-T developers that has attracted the likes of Novartis, Gracell, AstraZeneca and Autolus. Investors focused on oncology might ask how much mileage remains in studying ADI-001 in haematological cancers, given that its development in diffuse large B-cell lymphoma (DLBCL) is being stopped.
To be clear, Adicet isn’t giving up on cancer. But it is scaling back ADI-001, a gamma-delta Car-T cell therapy targeting CD20, whose phase 1 Glean-1 trial has enrolled mostly DLBCL patients; Adicet today said it would focus only on mantle cell lymphoma, a much rarer and arguably less aggressive type of non-Hodgkin’s lymphoma than DLBCL.
Worsening dataset
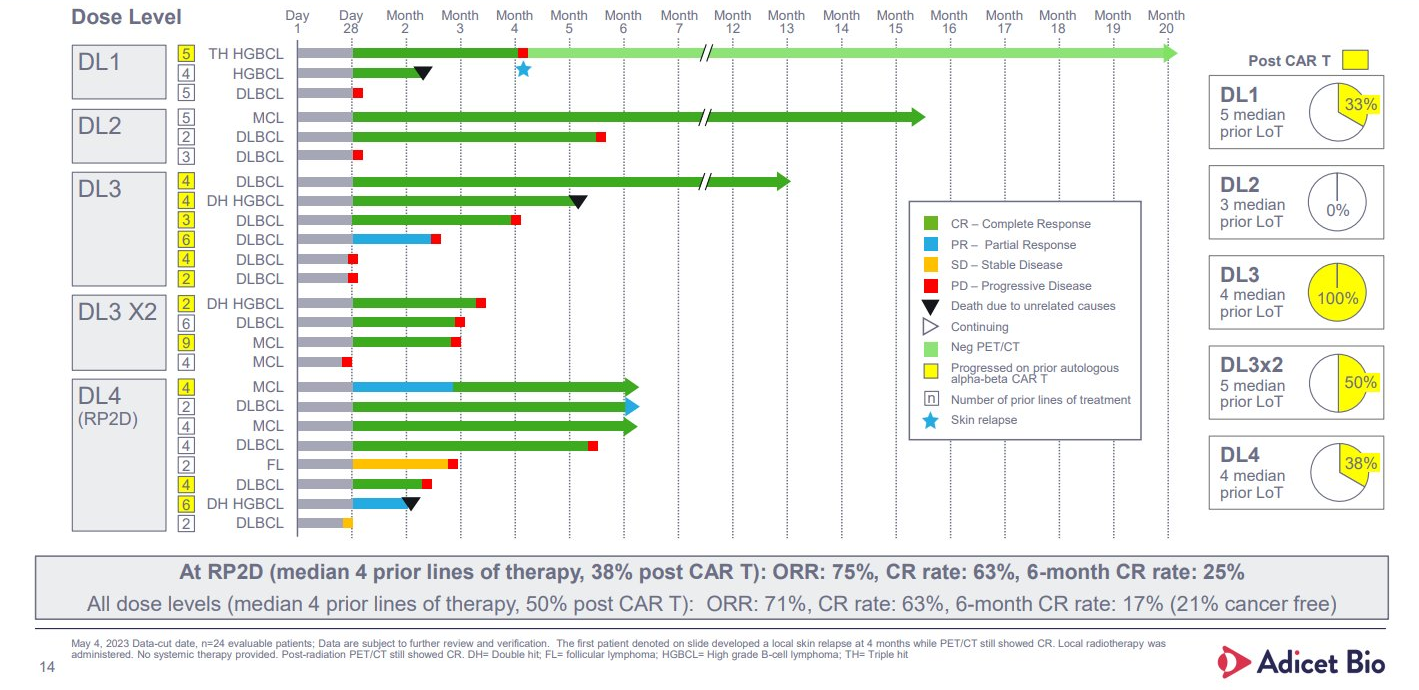
Investors will note that the Glean-1 dataset, which has been through four iterations since first being presented at ASCO in 2022, has been worsening as more patients have been enrolled and followed up.
A 75% complete remission rate in the first eight subjects fell to 63% at the last update, in June 2023, while the best overall response rate stands at 71% across 24 patients. That’s still very respectable in a relatively aggressive lymphoma patient population, but what has caused concern is ADI-001’s lack of durability – the same problem that hit allogeneic Car-T players.
Indeed, looking only at ADI-001 patients with at least six months’ follow-up, the ORR is only 21%, including one complete remission that worsened to a partial response. The full dataset comprises four dose levels, and it’s also notable that hopes that double-dosing ADI-001 would improve durability have evaporated: all three patients initially responding to this relapsed within four months.
So what’s the evidence backing mantle cell lymphoma? So far there are only five efficacy-evaluable patients with this malignancy in Glean-1, and that’s from a dataset last updated seven months ago.
Four of these subjects yielded complete remissions, and while one relapsed early the other three were last reported to be ongoing at six to 15 months; the recommended phase 2 dose level 4 includes two mantle cell patients, both in complete response at six months at least year’s update.
The last reported data from Glean-1
Interestingly, on an analyst call today Adicet denied that ditching the DLBCL indication was the result of data it had seen in Glean-1, saying instead that this decision was “purely budget driven”. It also mooted the possibility of looking at another relatively unaggressive malignancy, follicular lymphoma, but for now the focus for ADI-001 is mantle cell.
A phase 2 study at dose level 4 (1 billion Car-positive cells) is being evaluated in third-line mantle cell lymphoma, and the aim would be for this to serve as the basis for an accelerated US approval, Adicet said. Meanwhile, Glean-1 will continue enrolling mantle cell patients, and the company promises an update in the second half of this year.
This morning Adicet stock climbed 25%, but the group is still trading well below its last reported cash balance of $183m, and it remains a biotech with much still to prove.
1337













